Cord Blood Potency Testing
Umbilical cord blood (CB) is a rich source of primitive hematopoietic stem and progenitor cells (HSPCs) for research and transplantation. However, the number of HSPCs in an individual CB unit is often limited by various factors, including unit volume. Cell processing and cryopreservation can also adversely affect the number and quality of viable stem and progenitor cells. For these reasons, it is critical to determine the тАЬpotencyтАЭ of CB units using a cord blood potency assay that measure the number of viable HSPCs.
We have compiled a selection of scientific resources below to help you with CB processing and potency testing.
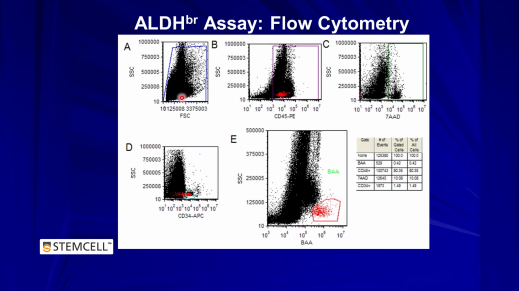
Development and Validation of a Rapid ALDHbr-Based Cord Blood Potency Assay
Join Dr. Kevin Shoulars, from Duke University Medical Center, as he discusses his work with Dr. Joanne Kurtzberg on the development of a rapid flow cytometry-based method for measuring the potency of cord blood units.
View Now >-
 Assays for Hematopoietic Stem and Progenitor Cells in Cord BloodThis webinar, by Dr. Bert Wognum, will provide an overview of the value of assessing hematopoietic progenitor cell content in cord blood. In addition to reviewing the current assays used, new methods incorporating automation will be discussed.
Assays for Hematopoietic Stem and Progenitor Cells in Cord BloodThis webinar, by Dr. Bert Wognum, will provide an overview of the value of assessing hematopoietic progenitor cell content in cord blood. In addition to reviewing the current assays used, new methods incorporating automation will be discussed. -
 How to Set Up the CFU Assay for Custom Hematopoietic Training Courses Using SmartDishтДвLearn how to perform all steps involved in the colony-forming unit (CFU) assay setup for custom hematopoietic training courses using SmartDishтДв cultureware
How to Set Up the CFU Assay for Custom Hematopoietic Training Courses Using SmartDishтДвLearn how to perform all steps involved in the colony-forming unit (CFU) assay setup for custom hematopoietic training courses using SmartDishтДв cultureware -
 Maintaining Humidity in CFU Assays using MethoCultтДв MediaTechnical tip from our dedicated team of Product and Scientific Support specialists
Maintaining Humidity in CFU Assays using MethoCultтДв MediaTechnical tip from our dedicated team of Product and Scientific Support specialists -
 CFU Assay Instructions for Global Proficiency Testing ProgramsLearn to perform the colony-forming unit (CFU) assay for Proficiency Testing programs
CFU Assay Instructions for Global Proficiency Testing ProgramsLearn to perform the colony-forming unit (CFU) assay for Proficiency Testing programs -
 Identification of Colonies Derived from Human Hematopoietic ProgenitorsRepresentative colony images and tips for identifying progenitor subtypes in CFU assays
Identification of Colonies Derived from Human Hematopoietic ProgenitorsRepresentative colony images and tips for identifying progenitor subtypes in CFU assays -
 Human Hematopoietic Stem and Progenitor Cell PhenotypingOverview of subset surface markers, frequencies and assays for analysis
Human Hematopoietic Stem and Progenitor Cell PhenotypingOverview of subset surface markers, frequencies and assays for analysis